Describe How the Atoms in a Compound Are Held Together
The purpose of the comparison of hydrogen peroxide and water above was to show you that the atoms in a given compound are always combined in a fixed ratio. Each hydrogen atom is covalently bonded to the oxygen via a shared pair of electrons.

Covalent Bonding Lesson 1 Covalent Bonding Covalent Bonds Atoms Held Together By Sharing Electrons Mostly Formed Between Nonmetals Molecules Neutral Ppt Download
A continuum of bond polarities exist between the purely covalent bond as in H2 and ionic bonds.

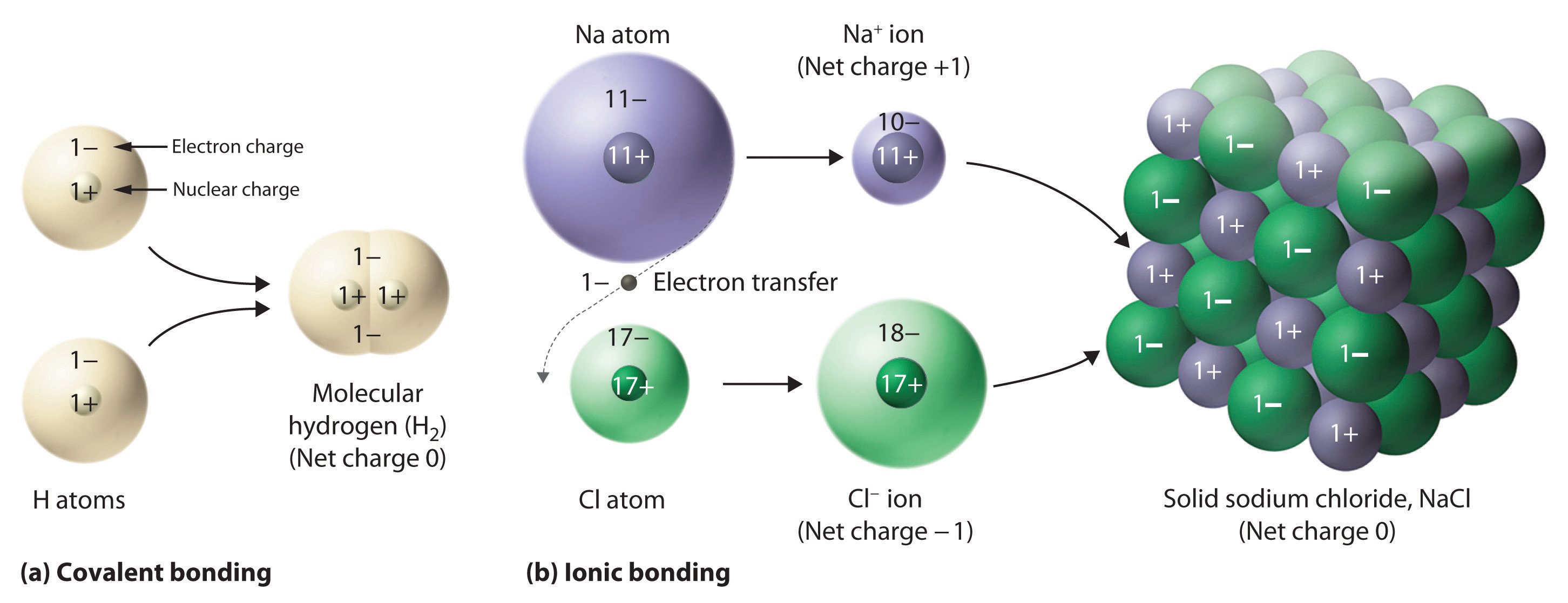
. These bonds form as a result of the sharing or exchange of electron s among the atoms. This was the fifth of Daltons postulates. 1 In chemistry a compound is a substance that results from a combination of two or more different chemical element s in such a way that the atom s of the different elements are held together by chemical bonds that are difficult to break.
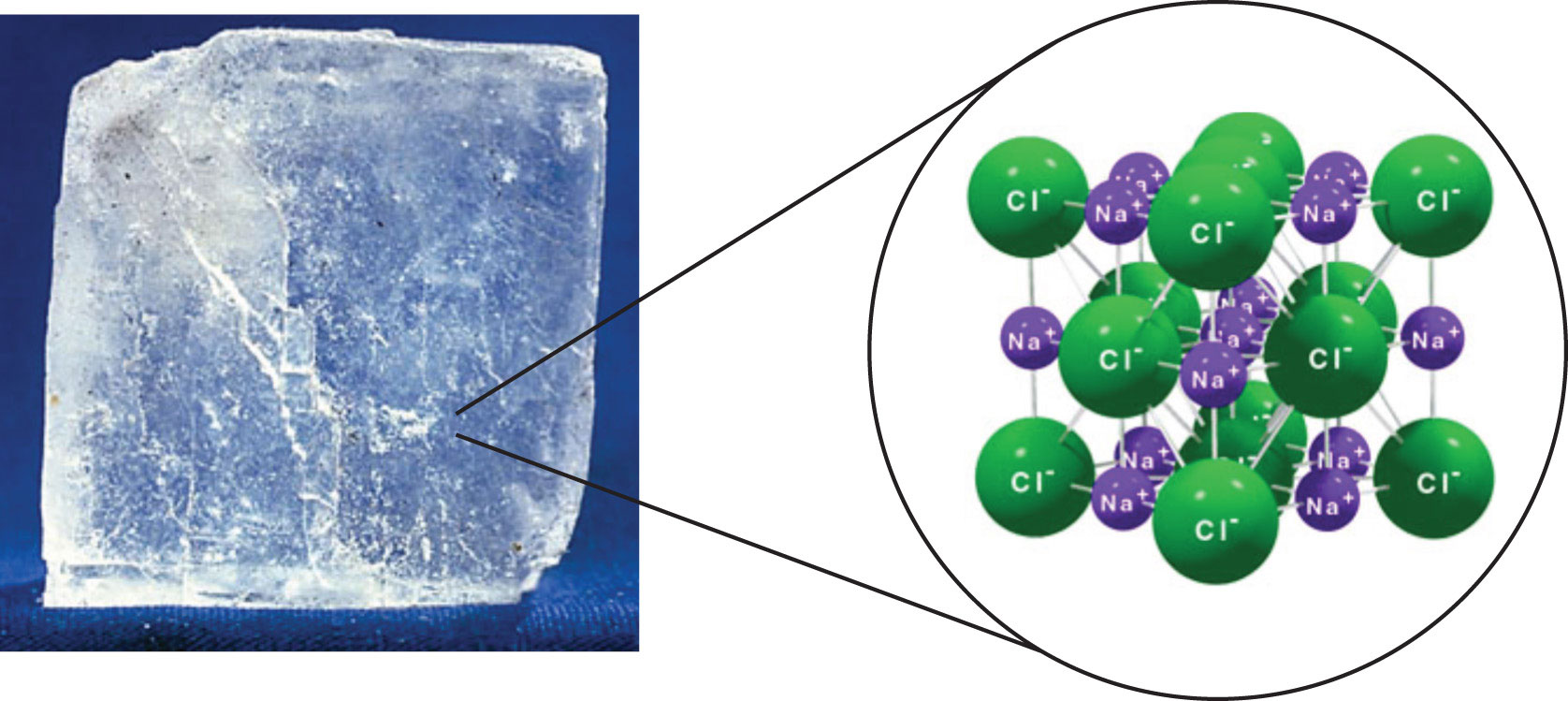
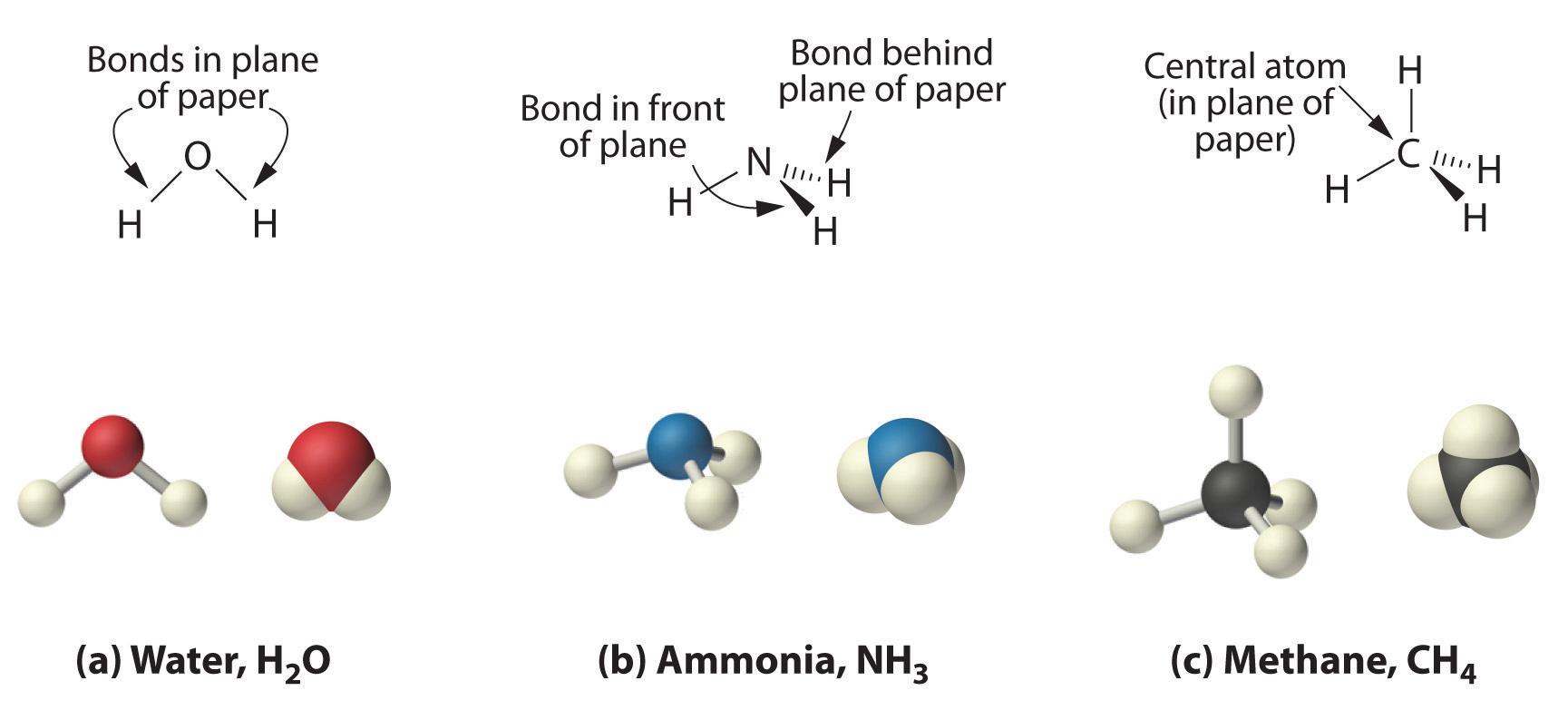
For example water H2O is a compound consisting of two hydrogen atoms bonded to an oxygen atom. Sodium chloride is an example of an ionic compound. The white stuff we know as sugar is sucrose a molecule composed of 12 atoms of carbon 22 atoms of hydrogen and 11 atoms of oxygen C 12 H 22 O 11.
Also learn about trends in the periodic table of elements and explore how the structure. Ate is employed when there are more oxygen atoms present in a compound and ite is used when number of oxygen atoms present in a compound is less. It is composed of one oxygen atom and two hydrogen atoms.
Atoms chemically combine in fixed. Thus there are 4 pairs of electrons surrounding the oxygen atom two pairs involved in covalent bonds with hydrogen and two unshared pairs on the opposite side of the oxygen. The last three alphabets of the non-metal are replaced with ide.
Oxygen also has two unshared pairs of electrons. Like all compounds made from these three elements sugar is a carbohydrate. Its found naturally in most plants but especially in sugarcane and sugar beetshence their names.
In all water molecules in the universe there will always be one O atom and two H atoms bonded together. Investigate how the transfer of electrons between atoms creates ions and how the mutual attraction of these charged particles forms ionic bonds. Is water ionic bond.
This interactive activity from ChemThink discusses ionic bondinga type of chemical bond formed between two ions with opposite charges. For example H2O is held together by polar covalent bonds. B If the compound contains polyatomic ion then the last three alphabets of a non-metal are replaced with ate or ite.

Nomenclature Ionic Compounds Held Together By Ionic Bonds What Are Ionic Bonds Between Metals And Non Metals Transfer Of Electrons Between Atoms Ppt Download
Molecules And Compounds Overview Atomic Structure Article Khan Academy
No comments for "Describe How the Atoms in a Compound Are Held Together"
Post a Comment